INTRODUCTION
To function as a living organism, the expenditure of energy is required. But how many of us truly understand exactly where this energy comes from? Does it come from the food we eat? Or is it simply a phenomenon of divine creation requiring no intuitive thought? Energy is a product, in the form of ATP (adenosine triphosphate), of an extensive number of chemical reactions that take place in a living organism. ATP can be synthesized through three primary pathways, but the scope of this paper will only extend to the phosphocreatine (creatine phosphate) pathway of ATP production in skeletal muscles.
Understanding how and why phosphocreatine works is of significance to anyone who makes use of cellular energy and particularly to persons who participate in any form of exercise. The role of phosphocreatine in skeletal muscle metabolism and resistive exercise is an interesting but somewhat controversial topic, which needs to be explored in order to understand how muscles work.
Creatine phosphate (PCr) is a high-energy phosphate compound synthesized in the liver and is instrumental in the synthesis of ATP (Brody, 1994). This compound (PCr) is used in the synthesis of ATP through an anaerobic pathway (requiring no oxygen) during high-intensity exercise and requires the presence of an enzyme known as creatine phosphokinase (CPK) or creatine kinase (CK) (Hochachka, 1994). The reaction by which PCr is used to produce ATP is as follows: PCr + ADP + H <=> ATP + creatine (Hochachka, 1994, and Trump, Hanstock, Allen, Gheorghiu, and Hochachka, 2001). This type of reaction is known as a feedback reaction, meaning that excess production of the end product would slow the reaction. Vicini and Kushmerick (2000) state that evidence exists to support the idea of a feed-forward reaction involving calcium activation. CPK acts on PCr to liberate the attached phosphate group allowing it to bond with ADP to form ATP in a process known as phosphorylation (Brody, 1994). Decking, Alves, Wallimann, Wyss, and Schrader refer to this process as the "buffer function" of CK (C320, 2001). They propose an alternate function of CK to facilitate production of energy within the cell, or "creatine kinase shuttle hypothesis" (Decking et. al., C320, 2001).
 The purpose of this paper is to explore the normal functioning of phosphocreatine in skeletal muscle metabolism and contraction, its association with and role in muscle fatigue during resistive exercise, and the effects of oral supplementation of creatine on skeletal muscle metabolism relative to resistive exercise.
The purpose of this paper is to explore the normal functioning of phosphocreatine in skeletal muscle metabolism and contraction, its association with and role in muscle fatigue during resistive exercise, and the effects of oral supplementation of creatine on skeletal muscle metabolism relative to resistive exercise.
I. Function of Phosphocreatine in Skeletal Muscle Metabolism
In order for skeletal muscles to carry out basic subsistence metabolism such as protein synthesis and active transport ATP is required. However, ATP is not available to the muscle cells in sufficient quantities for sustained metabolism and contraction during intense periods of resistive exercise due to the fact that reserves of this compound are rapidly depleted. First, primary sources of energy such as glucose, glycogen and fatty acids must be properly catabolized to release the high-energy phosphate ATP, which is required for metabolism. As mentioned earlier, phosphocreatine is used for metabolism when there is an insufficient amount of oxygen available (anaerobic) as in high-intensity exercise. Thus, the phosphate group of PCr is liberated by the action of the enzyme CPK and is bound to ADP, resulting in the production of ATP and "free" creatine (Cr) (Hochachka, 1994).
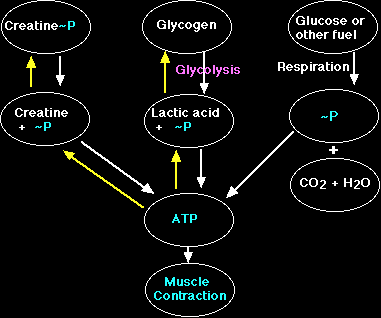
A Breakdown Of Creatine
Cr is responsible for acid-base regulation, protein synthesis, and providing skeletal muscle energy (Murphy, McConell, Cameron-Smith, Watt, Ackland, Walzel, Wallimann, and Snow, 2001). This process takes place within the muscle cell's mitochondria, and the resulting ATP is then transported to the proteins in the sarcomere of the myofibril, which are responsible for contraction (Brody, 1994). According to Murphy et al. (2001) Cr is regulated by a specific transporter protein (CreaT) located on the sarcollema. The activity of this protein, in turn, is regulated by sodium and chloride transporters (Murphy et al., 2001). Also, the content of Cr in the muscle is dependent upon the intake/efflux of Cr (Murphy et al., 2001).
Metabolism of phosphocreatine in the anaerobic environment ultimately results not only in the production of ATP but also in the production of lactic acid (Schneck, 1992). Despite the temporary replenishing of ATP concentrations resulting from phosphocreatine phosphorylation, skeletal muscles are still limited in their overall production due to the rapid depletion of PCr during intense exercise (Greenhaff, Bodin, Sodurlund, and Hultman, 1994). According to Chung, Shawman, Carlsen, Unger, Larson, and Jue (1998) the amount of ATP hydrolyzed should equal the amount of energy released in the form of heat and total work. However, these authors state that the amount of energy produced exceeds the amount of available energy from ATP hydrolysis indicated by a fall in PCr levels (Chung, et al., 1998).
II. Function of Phosphocreatine in Skeletal Muscle Contraction
Phosphocreatine has been found to play a significant role in the processes associated with the contraction of skeletal muscles (Greenhaff, et al., 1994). To gain a greater understanding of exactly how phosphocreatine works in contraction, one must first understand the mechanisms of contraction.
A) Mechanisms of Contraction
In order for a skeletal muscle to contract, it must first receive an action potential from an efferent neuron, which innervates a motor end plate within the muscle fiber, great enough to cause a contraction. This action potential is then carried into the belly of the fiber to the myofibrils via transverse tubules. The depolarization resulting from the action potential causes calcium ions to be released from the terminal cisternae via the sarcoplasmic reticulum (Fitts, 1994).
After being liberated from the sarcoplasmic reticulum, the calcium ions diffuse through the cytoplasm and bond to the protein troponin, which is found on the actin filament of the sarcomere and blocks the binding sites between the actin and the globular head of the myosin. When the calcium bonds to the troponin, it causes this protein to move away from the binding site thus allowing the globular head of the myosin filament to bond to actin. The cross-bridge formed between the actin and myosin filaments then goes through an ATP power stroke cycle, which causes the sarcomere to contract. These two structures remain connected until ATP breaks the bond between them, causing the globular head to prepare for another contraction, thus relaxing the sarcomere. Another point to keep in mind is muscle recruitment. Muscles are recruited in order based on their size; smaller type I fibers are recruited before the larger, more powerful type II fibers (Blei, Conley, and Kushmerick, 1993).
Chung et al., raise the question regarding the breakdown of PCr before the muscle contraction occurs, or if this process takes places during the contraction or relaxation of the muscle (1998). These authors found that "the PCr level falls rapidly within 16 ms after stimulation and exhibits a kinetics curve that mirrors the force development profile (Chung, et. al., C846-C847, 1998).
B) Contraction in the Absence of Phosphocreatine
The role of phosphocreatine in the series of events leading to muscle contraction is to allow for the formation of the ATP molecule when the stored supply has been depleted. Without the presence of PCr and its ability to maintain an adequate concentration of ATP in the muscle fiber, the myosin would therefore be unable to release from the actin resulting in either muscle fatigue or a state similar to rigor mortis (Brody, 1994). According to one group of authors, even in a low concentration of PCr, the CK flux remains higher than the amount of ATP synthesized in the body (Stepanov, Mateo, Gillet, Beloeil, Lechene, and Hoerter, 1997). This indicates PCr is not the limiting factor in ATP synthesis.

III. Association of Phosphocreatine Levels with Muscle Fatigue During Resistive Exercise
Muscle fatigue is explained by Schneck (1992) as being the point at which the demand for energy required by the muscle exceeds the level available either from the stored supply of ATP or the indirect synthesis of high-energy ATP through phosphocreatine phosphorylation. Trump et al. (2001) described a decrease in peak intensity from ischemic fatigue that slowly recovers to only 75-80% of resting peak intensity. As a result of the absence of sufficient amounts of phosphocreatine, the muscle, through the anaerobic pathway, creates a by-product known as lactic acid (Schneck, 1992). The presence of lactic acid, therefore, is the ultimate cause of muscle fatigue according to these authors. As a result of the accumulation of lactic acid in the muscle, the performance of the muscle is drastically decreased (Fitts, 1994). This decrease in performance is attributed to consequences such as limited output and force exerted by the muscle and is dependent upon conditions such as inherent muscle type and duration of exercise (Fitts, 1994).
In opposition of this theory, Bangsbo, Graham, Kiens, and Saltin (1992) state that "muscle and blood lactate concentrations are not the only crucial factors for inhibiting anaerobic carbohydrate usage during intense skeletal muscle contractions and the development of fatigue" (p. 206). Bangsbo et. al., state that fatigue during resistive exercise is primarily dependent upon usage of stored glycogen in the working muscles (1992). They continue to describe that exhaustion is postponed relative to the availability of stored glycogen and that fatigue occurs when these stores are completely depleted (Bangsbo et al., 1992). According to these authors, a linear relationship exists between glycogen utilization and lactate accumulation during exercise bouts of 15 minutes (Bangsbo et. al., 1992).
Petersen, Ostrowski, Ibfelt, Richelle, Offord, Kristensen, and Pedersen (2001) explain that exercise causes a 20-fold increase in oxygen consumption that increases the production of free radicals. These free radicals cause damage to cellular membranes, DNA, and protein and carbohydrate structures (Petersen et al., 2001). This destruction of cellular constituents is evidenced by an increased presence of CPK. Remember that CPK is the enzyme that breaks the bond holding PCr together, thereby releasing a phosphate group. One must keep in mind that most resistive exercise regimens do not last for 15 minutes. Most high intensity resistive exercise bouts are short-term and repetitive. Preen, Dawson, Goodman, Lawrence, Beilby, and Ching performed a study utilizing measurements of ten repetitions lasting six seconds (2001). These authors found that muscles rely more on aerobic production of ATP for use in contractions over a longer period of time (Preen et. al., 2001). This increased demand of oxygen during resistive exercise is secondary to rapid depletion of PCr levels (Preen et. al., 2001). The focus of their paper is discussed later.
IV. Metabolic Effects of Oral Creatine (Cr) Supplementation
Although phosphocreatine does exist naturally in the body, oral supplementation of elemental creatine (Cr) has been shown to have an effect on phosphocreatine levels as well as skeletal muscle metabolism (Greenhaff et. al., 1994). In a recent study by Greenhaff et. al. (1994), these authors demonstrated that taking creatine orally, either in natural forms such as meat or as nutrient supplements, does aid skeletal muscle metabolism by increasing the amount of phosphocreatine levels present in the muscle fibers.
This study involves male volunteers who were willing to supplement their diets with creatine and participate in various forms of exercise. Measurements of isometric force of the knee extensors of each subject were recorded to serve as a basis by which to compare pre-ingestion and post-ingestion levels of phosphocreatine in the skeletal muscles receiving the exercise (Greenhaff et. al., 1994). Electrical stimulation was also applied to the subjects' knee extensor muscles to cause contraction. Biopsies of the muscle of each subject were taken before creatine supplementation and ten days later after a specified amount of ingested oral creatine in attempts of demonstrating a difference in phosphocreatine levels between the two periods (Greenhaff et. al., 1994). The biopsies were then desiccated and the concentrations of such compounds as ATP and phosphocreatine were calculated through use of a spectrophotometer (Greenhaff et. al., 1994).
 As a result of oral supplementation of creatine, Greenhaff et al (1994) found that levels of phosphocreatine and ATP were higher during the recovery period between exercise and electrical stimulation after ingestion of the creatine (Figure 3). To see their results, refer to Figure 3. The increase in concentration levels of phosphocreatine, therefore, would allow the skeletal muscles to continue their metabolic and contractile processes (in an anaerobic state) for a limited period of time.
As a result of oral supplementation of creatine, Greenhaff et al (1994) found that levels of phosphocreatine and ATP were higher during the recovery period between exercise and electrical stimulation after ingestion of the creatine (Figure 3). To see their results, refer to Figure 3. The increase in concentration levels of phosphocreatine, therefore, would allow the skeletal muscles to continue their metabolic and contractile processes (in an anaerobic state) for a limited period of time.
Greenhaff et al. (1994) concluded that the ingestion of an increased amount of creatine did in fact augment the capacity for work done by skeletal muscles. The researchers attributed this modification to the fact to the potential for accelerated levels of creatine to offset the reaction by which phosphocreatine is involved in the production of ATP (Greenhaff et. al., 1994).
According to Rush, Tullson, and Terjung (1998) "the decrease in phosphocreatine (PCr) that occurs during intense contractions of fast-twitch muscle impairs the ATP-buffering role of creatine kinase (CK)" (C465). One can therefore infer that by increasing the amount of Cr available to the working muscles, one can enhance the levels of ATP.
In another study by Balsom et. al. (1995), it was found that oral supplementation of creatine allows skeletal muscles to maintain a higher power output during short period of high-intensity exercise. The study was executed by having subjects pedal a bicycle at a certain frequency over a period of 6 seconds (Balsom et. al., 1995). Blood samples and muscle biopsies were then taken from each subject to measure levels of phosphocreatine, ATP and lactic acid.
As a result of their study, Balsom et al. (1995) found that, after oral creatine supplementation, levels of phosphocreatine were higher and lactic acid concentrations were lower as opposed to measurements taken before creatine ingestion. Thus, it was suggested that creatine supplementation accounts for a faster breakdown of phosphocreatine which, in turn, accelerated the production of ATP during high-intensity exercise (Balsom et al., 1995).
Most authors agree that the appropriate amount of creatine for supplementation is 20 grams per day (Preen et al., 2001, and Birch, Noble, and Greenhaff, 1994). Birch et al., used this rate of creatine supplementation over a period of five days. They found that this regimen increases the Cr concentration in skeletal muscle by 25 mmol/kg, of which 8mmol/kg is PCr (Birch et al., 1994). "This regimen of Cr feeding can also decrease the loss of muscle force production and the accumulation of plasma ammonia" (Birch et al., p 268, 1994). These authors state that the ingestion of Cr increased mean peak output of their experimental group by approximately 6% (Birch et al., 1994).
DISCUSSION
Phosphocreatine is a compound instrumental in the synthesis of high-energy ATP (Brody, 1994). It also plays a major role in the functioning of skeletal muscle metabolism and contraction. When phosphocreatine quantities are exhausted and ATP is no longer being produced through this pathway, lactic acid concentrations rise causing the muscles to become fatigued (Fitts, 1994). In the absence of adequate PCr, the body shifts pathways from the anaerobic pathway to the aerobic pathway to provide the requisite ATP (Preen et al., 2001).
Researchers found through scientific studies that ingestion of creatine allows for a greater capacity of work to be done by skeletal muscles (Greenhaff et al., 1994, Preen et al., 2001, and Balsom et al., 1995). This elevated capacity of work exhibited during short bouts of high-intensity exercise is attributed to lower concentrations of lactic acid, blood lactate, and elevated levels of ATP.
In conclusion, normal functioning of skeletal muscles consists of a highly complex and extensive series of chemical reactions and interactions. It is fundamental for persons to have a basic knowledge of compounds such as phosphocreatine and ATP in order to answer questions pertaining to human physiology.
LITERATURE CITED
Balsom, P. D., K. Sodurlund, B. Sjodin, and B. Ekblom. Skeletal muscle metabolism during short duration high-intensity exercise: influence of creatine supplementation. Acta Physiologica Scandinavica, vol.154, 303-310, 1995.
Bangsbo, J., Gollnick, P., Graham, T., and Saltin, S. Substrates for muscle glycogen synthesis in recovery from intense exercise in man. Journal of Physiology, vol. 434, 423-440, 1991.
Bangsbo, J., Graham, T., Kiens, B., and Saltin, B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. Journal of Physiology, vol. 451, 205-227, 1992.
Birch, R., Noble, D., and Greenhaff, P. The influence of dietary creatine supplemenation on performance during repeated bouts of maximal isokinetic cycling in man. European Journal of Applied Physiology, vol. 69, 268-270, 1994.
Blei, M., Conley, K., and Kushmerick, M. Separate measures of ATP utilization and recovery in human skeletal muscle. Journal of Physiology, vol. 465, 203-222, 1993.
Brody, T. Nutritional Biochemistry. Academic Press, San Diego, California. 1994.
Chung, Y., Sharman, R., Carlsen, R., Unger, S., Larson, D., and Jue, T. Metabolic fluctuation during a muscle contraction cycle. American Journal of Physiology, vol. 274, C846-C852, 1998.
Decking, U., Alves, C., Wallimann, T., Wyss, M., and Schrader, J. Functional aspects of creatine kinase isoenzymes in endothelial cells. American Journal of Physiology and Cellular Physiology, vol. 281, C320-C328, 2001.
Fitts, R. H. Cellular mechanisms of muscle fatigue. Physiological Reviews, vol. 74, 49-95. 1994.
Greenhaff, P. L., K. Bodin, K. Sodurlund, and E. Hultman. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. American Journal of Physiology, vol. 266, E725-E730. 1994.
Hochachka, P. Muscles as Molecular and Metabolic Machines. CRC Press, Boca Raton, Florida. 1994.
Murphy, R., McConell, G., Cameron-Smith, D., Watt, K., Ackland, L., Walzel, B., Wallimann, T., and Snow, R. Creatine transporter protein content, localization, and gene expression in rat skeletal muscle. American Journal of Physiology and Cellular Physiology, vol. 280, C415-C422, 2001.
Petersen, E., Ostrowski, K., Ibfelt, T., Richelle, M., Offord, E., Kristensen, J., and Pedersen, B. Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. American Journal of Physiology and Cellular Physiology, vol.280, C1570-1575, 2001.
Preen, D., Dawson, B., Goodman, C., Lawrence, S., Beilby, J., and Ching, S. Effect of creatine loading on long-term spring exercise performance and metabolism. Medicine & Science in Sports & Exercise, vol. 33, 814-821. 2001.
Rush, J., Tullson, P., Terjung, R. Molecular and kinetic alterations of muscle AMP deaminase during chronic creatine depletion. Cellular Physiology, vol. 43, C465-C471.
Schneck, D. J. Mechanics of Muscles: Second Edition. New York University Press, New York and London. 1992.
Stepanov, V., Mateo, P., Gillet, B., Beloeil, J., Lechene, P., and Hoerter, J. Kinetics of creatine kinase in an experimental model of low phosphocreatine and ATP in the normoxic heart. American Journal of Physiology, vol. 273, C1397-C1408, 1997.
Trump, M., Hanstock, C., Allen, P., Gheorghiu, D., and Hochachka, P. An 1H-MRS evaluation of the phosphocreatine/creatine pool (tCr) in human muscle. American Journal of Physiology and Regulatory Integrative Comparitive Physiology, vol. 280, R889-R896, 2001.
Vicini, P., and Kushmerick, M. Cellular energetics analysis by a mathematical model of energy balance: estimation of parameters in human skeletal muscle. American Journal of Physiology and Cellular Physiology, vol. 279, C213-C224, 2000.
