Amino acids are the "building-blocks" of proteins. Proteins, from the Greek word meaning "of prime importance," constitute an array of structures. Examples of these structures include hormones, enzymes, and muscle tissue.
The primary function of protein is growth and repair of body tissue (anabolism). Proteins can also be used as energy through catabolic (breakdown of tissues) reactions, such as gluconeogenesis-the process of making glucose from amino acids, lactate, glycerol, or pyruvate in the liver or kidneys.
Our current examination of proteins and amino acids will cover the metabolism of the protein we eat, dietary protein, and catabolic situations in the body. A general understanding of the molecular structure of proteins and amino acids is needed to understand their metabolism.
The Structure Of Proteins & Amino Acids
Protein is comprised of carbon, hydrogen, oxygen and, most importantly, nitrogen. Protein may also contain sulfur, cobalt, iron, and phosphorus. These elements form the "building blocks" of protein, amino acids. A protein molecule is made up of long chains of amino acids bonded to each other by amide bonds, or peptide linkages.
The food (protein) we eat contains different amino acids depending on the type of amino acids present. An almost endless combination of amino acid bonds can exist. The combination of amino acids governs the protein's properties.
Just as the combination of amino acids governs the specific proteins properties, the structure of individual amino acids determines its function in the body. An amino acid is made up of a central carbon atom, a positively charged amine group (NH2) at one end and a negatively charged carboxylic acid group at the other (COOH). Another group termed the R GROUP or side chain determines the function of the amino acid. The side chain varies among the different amino acids.
Our bodies require 20 different amino acids. These amino acids can be divided into many groups based on their physical properties. For the purposes of our discussion there are two relevant groups:
- Essential amino acids (EAA)
- Nonessential amino acids (NEAA).
EAA must be consumed through ones diet, because they cannot be synthesized in the body at a sufficient rate to meet demands. NEAA are not nonessential meaning they can be synthesized in the body from other protein and non-protein nutrients, not that they are less important than the EAA.
| Essential Amino Acids | Nonessential Amino Acids |
| Histidine | Alanine |
| Isoleucine | Arginine |
| Leucine | Aspartic Acid |
| Lysine | Cysteine |
| Methionine | Cystine |
| Phenylalanine | Glutamic Acid |
| Typrophan | Glutamine |
| Valine | Glycine |
| Proline | |
| Serine | |
| Threonine | |
| Tyrosine |
Food (protein) that contains all of the EAA is termed a complete protein. Food that does not contain all of the EAA is termed an incomplete. Combining two or more incomplete proteins, if they together supply all the EAA, can make a complete protein.
Ingestion & Digestion
Dietary protein must first be broken down into peptide fragments. This breakdown is accomplished in the stomach, by pepsin, and in the small intestine, by chymotrypsin and tyrpsin (pancreatic enzymes).
These peptide fragments now must be broken down into free amino acids (not linked to other amino acids). This breakdown is accomplished by aminopeptidase, located on epithelial cells of the small intestine, and carboxypeptidase, from the pancreas.
The now free amino acids are carried into the epithelial cells by secondary active transport coupled with sodium. Short chains of amino acids, di- or tripeptides, can be absorbed by secondary active transport coupled to the hydrogen ion (H+) gradient.
There are different transporters for specific amino acids. Once in the epithelial cell, these small peptides are hydrolyzed (broken down) into amino acids. Both types of absorption require ATP. Next the free amino acids enter the blood through a facilitated diffusion carrier in the cell membrane.
 |
What Is ATP? A nucleotide, C10H16N5O13P3, that contains high-energy phosphate bonds and is used to transport energy to cells for biochemical processes, including muscle contraction and enzymatic metabolism. |
 |
 |
||
These amino acids in the blood and extracellular fluids make up a large group called the amino acid pool. This pool also contained amino acids that were catabolized from other tissues and those created in the liver. Amino acids are constantly entering and leaving this pool.
 |
||||
 |
|
 |
||
 |
||||
Absorbed amino acids enter one of two places, the liver or other cells. Those that enter the liver are either used to synthesize proteins or converted to -ketoacids, carbohydrate-like intermediates, through the process of deamination.
Deamination
Since the body cannot obtain usable energy from the nitrogen in amino acids, the nitrogen must be removed before the carbon skeleton, -ketoacids, can be used. Deamination involves removing the amino group from amino acids. The nitrogen from these amino groups is transfer to glutamate, which can then be released as ammonia in the glutamate dehydrogenase reaction.
This removed nitrogen is used to form urea in the liver, which is sent to the kidneys to be excreted.
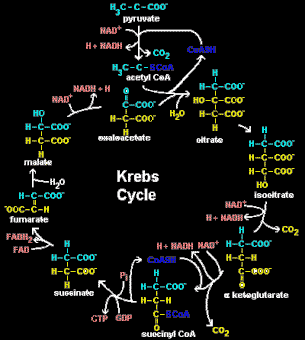
The Kreb Cycle
The remaining -ketoacids can provide energy for the liver by being catabolized in the Krebs cycle, used to create glucose through gluconeogenesis, or used for fat synthesis by providing acetyl-CoA (a substrate to synthesize fatty acids). They can also be converted to a new amino acid through transamination.
 |
What Is The Kreb Cycle? In all plants and animals, a series of enzymatic reactions in the mitochondria of cells, used to produce high-energy phosphate compounds that are the source of cellular energy. |
 |
 |
||
Transamination
Transamination involves transferring and amino group to another -ketoacids. Most transaminase reactions involve transferring an amino group to -ketoglutarate, forming a new -ketoacid and glutamate.
An important transaminase reaction involves the branched chain amino acids (BCAA), which occurs primarily in muscle. In this reaction, the amino groups of the BCAA are removed and transferred to -ketoglutarate, which forms branched chain keto acids (BCKAs) and glutamic acid (glutamate).
The amino group on glutamic acid is then transferred to pyruvate, which creates -ketoglutarate and alanine. The alanine is sent from the muscle to the liver, where the amino group is removed from it and transferred to oxaloacetate, remaking -ketoglutarate and pyruvate.
This pyruvate, which is now in the liver, can be used to make glucose. This is called the glucose-alanine cycle. During exercise this process is accelerated. To meet the demands of exercise muscle protein is broken down to deliver the needed BCAA for the glucose-alanice cycle. This is an example of protein turnover.
Protein Turnover & Nitrogen Balance
Amino acids taken up by cells are used to synthesis proteins. All cells require a constant supply of protein as they are always in constant flux of protein turnover. Protein turnover is composed of two parts: proteins synthesis and protein breakdown.
The largest amount of body protein is in the form of muscle. When amino acid requirements are not met, muscle is broken down into amino acids, which are then sent to the amino acid pool to be used accordingly. When more protein is broken down than is synthesized, one loses protein.
The opposite is true when one synthesizes more protein than is broken down, he/she loses protein. Without a sufficient intake of protein (malnutrition), one could not meet the demands of protein turnover and would eventually die.
In order to meet the demands of tissue breakdown in the body, the body needs new amino acids. Dietary protein is our primary source of amino acids. Due to this well known importance, of the three macronutrients, carbohydrate, fat, and protein, protein is the only one with a recommended daily allowance (RDA). The current RDA of sedentary adults is 0.83 grams of protein per kilogram of bodyweight (0.377 grams per pound of bodyweight).
Despite debate about the RDA and exactly how much protein should be consumed, it should be obvious that anyone who exercises, who is increasing the amount of protein broken down, needs more protein than a sedentary person.
Remember, protein turnover = protein synthesis - protein breakdown. If one is looking to gain muscle, then protein turnover needs to be positive, or is positive nitrogen balance. The term nitrogen balance is used as a measure of nitrogen (protein) intake and excretion. The letter N is used to symbolize nitrogen.
When this equation equals 0, one is said to be in nitrogen balance. When the equation is greater than zero, one is in positive nitrogen balance, and the additional protein is used to synthesize new tissues.
When the equation comes out to be less than 0, one is in a negative nitrogen balance and protein must be used for energy. This could lead to the use of amino acids from skeletal muscle.
The body does not store protein like it does with fat (adipose tissue) or glucose (glycogen), which are both easily accessible. Any ingested protein above what is needed to maintain protein turnover is converted to glucose or fatty acids.
Therefore, the body must breakdown functional tissue, skeletal muscle, to meet energy demands when is a negative nitrogen balance. In most cases, this does not pose much of a threat because the protein content of adult bodies is relatively constant and one would most likely oxidize an equal amount of amino acids as taken through dietary sources.
Athletes' protein content on the other hand is not constant due to protein breakdown caused by training.
Due to the strenuous workouts athletes often go through, many doctors and scientist now recommend a daily protein intake of 1.2 to 1.8 grams per kilogram of body weight.
Summary & Conclusion
- Amino Acids = C + NH2 + COOH + R Group
- Digestion - Protein » Peptide Fragments » Free Amino Acids
- Amino Acids can be used for: protein synthesis, energy production, gluconeogenesis, transamination, fat formation, or urea production.
- Protein turnover = protein synthesis (anabolic) - protein breakdown (catabolic)
- Nitrogen Balance = Nt (total intake) - Nu (in urine) - Nf (in feces) - Ns (in sweat)
References:
- Houston, Michael (2001). Biochemistry Primer for Exercise Science (2nd Ed.). Illinois: Human Kinetics
- Katch, Frank. Katch, Victor, McArdle, William (2001). Exercise Physiology: Energy, Nutrition, and Human Performance (5th Ed.). Maryland: Lippincott William and Wilkins.
- Widmaier, Eric. Raff, Hershal, Kevin, Strange (2004). Human Physiology: The Mechanisms of Body Function (9th Ed.) Boston: Mcgraw Hill.

